What’s the Deal with HCQ? Updated.
What does the Data on HCQ say?
Updated: October 23, 2020
????? ?? ? ??? ?? ????????? ?? ?????????????????? (???) ??? ??????? ?? ?????. Compounded with the new America’s Frontline Doctors group and the retracted Lancet study, I thought I could do an easy to understand review of the data so far, and why evidence is more than just data.
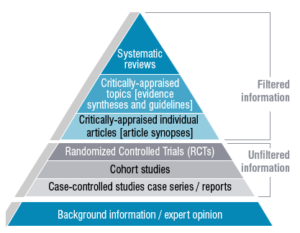
Image: University of Canberra Library
??? ????????? ?? ???????? ????? ???????? ???? ?????????? ??????? ??? ????-???????? ?? ??? ??? – this type of an exhaustively comprehensive publication goes through all the published studies on a topic, critically analyses all the data and then synthesizes the findings. This type of evidence takes into consideration the totality of data, taking into account not just what the data says – but how robust and rigorous the data and methodology is.
??? ???????? ????? ??? ???? ???????? ?? ??? ????? ???????????? ?? ?? ???? ?? ? ????????? ??? ?????-??. This was a large and robust randomized controlled trial led by the U.K. The study recruited a total sample size of 15,000 of which 1542 hospitalized patients were given HCQ and 3132 randomized to standard of care. This study showed us HCQ was not effective in reducing mortality, duration of hospitalization or any disease outcomes. In fact, they showed that HCQ was associated with an increased length of hospital stay and increased risk of progressing to invasive mechanical ventilation or death.
The SOLIDARITY trial conducted by the WHO evaluated the effects of four potential drug regimens for Covid-19 in 11,266 adult patients across more than 30 countries, one of which was HCQ. HCQ was found to have little or no effect on overall mortality, initiation of ventilation and duration of hospital stay in hospitalized patients.
.
On pre-exposure prophylaxis, a recent RCT was published on 1483 health care workers who were randomized to HCQ 1x or 2x weekly vs placebo. There was no difference in the development of confirmed or probably Covid-19.
??? ????? ???? ?????– a retrospective observational study where they reviewed medical records of 2451 patients who received treatment for Covid-19. Remember that this isn’t a study where they first recruited individuals, then randomized and gave them different medicines. They had no contact with these patients, simply looked back at their records. This study showed a decrease in mortality with HCQ. However, 77% of the patients in the group that received HCQ also received steroids (whereas only 36% received a steroid in the non-HCQ arm).
?? ??? ????? ?? ??? ???? ???, ????????? ?? ??????? ???? ????????? ?? ? ???? ?????????? ???????. ???? ?? ????? ??? ?????? ???????? ??? ???? ?????????? ???????? ??? ?? ?????? ???? ????? ?????? ????? ?? ???? ???????????. ???? ?? ? ???? ???? ?? ????? ?? ???????, ????????? ??? ????.
????????????? ??? ?????? ????? ?????? ??? ???? ???????????, ????????? ??? ???????????. It had to do with a company by the name of Surgisphere, who were collecting data for this study (and several others). When the publication came out in pre-print, there were several discrepancies in data. For example, in the study, data collected from patients in many countries exceeded the total number of Covid-19 patients in the country. To add to this, the company refused to release basic information, such as which hospitals participated in the study, citing contractual privacy. This is inherently incorrect because in typical situations, hospitals generally want to be recognized for their research (especially pivotal ones like this!) and are typically named in the publication as a general practice. They also named several European cities as sites of clinical trials, but when the major hospitals in these cities were contacted, all of them stated they did not participate in these studies. So, then that begs the question – where did this supposed data come from? And this, right here, is the reason why the study was retracted.
? ???? ?? ??? ???? ?? ??????? ??????? ????? ??? ??-?? ?? ?????? ????? ?? ????????. I won’t spend much time on this as Science Based Medicine has done a great deep dive on this. But three important things to remember:
